 COA COA |
 MSDS MSDS |
 HPLC HPLC |
 NMR NMR |
| CAS No: | 150322-43-3 |
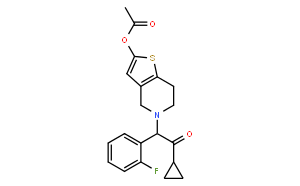
| Molecular formula(MF) | C20H20FNO3S |
| Molecular Weight(MW): | 373.44 |
| Alias | 5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl acetate; Acetic acid 5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl ester; CS 747; Ethanone, 2-(2-(acetyloxy)-6,7-dihydrothieno(3,2-c)pyridin-5(4H)-yl)-1-cyclopropyl-2-(2-fluorophenyl)-; Prasugrel [INN] |
| In vitro | DMSO | 30 mg/mL (80.33 mM) |
|---|---|---|
| Ethanol | 7 mg/mL (18.74 mM) | |
| Water | <1 mg/mL | |
| In vivo |
| Description | Prasugrel is a thienopyridine ADP receptor (P2Y12) antagonist, used for the reduction of thrombotic cardiovascular events. | |
|---|---|---|
| Targets |
|
|
| In vitro |
Prasugrel is a novel orally active thienopyridine with faster, higher and more reliable inhibition of platelet aggregation than clopidogrel reflecting its metabolism in vivo to an active metabolite with selective P2Y(12) antagonistic activity. [1] |
|
| In vivo | Prasugrel shows platelet inhibition that was 8.2 times more potent than clopidogrel in WT mice. [1] Prasugrel (3 and 10 mg/kg) dose-relatedly and significantly reduces thrombus-mediated cerebral infarction 24 hours after the irradiation in rat models of cerebral and peripheral arterial occlusive diseases. Prasugrel (0.3-3 mg/kg) reduces incidence, total area, and total number of cerebral infarcts in a dose-related manner 24 hours after the vascular injury in an embolic infarction rats model. Prasugrel (0.03-3 mg/kg/day) administered from the day before the lauric acid injection for 11 successive days inhibits the progression of the disease in a dose-related manner in rats with lauric acid-induced peripheral arterial occlusive diseases. [2] Prasugrel administrated in dogs (0.03-0.3 mg/kg/day) and monkeys (0.1 and 0.3 mg/kg/day) once a day for 14 days results in potent, dose-related and cumulative inhibition of ADP-induced platelet aggregation. Prasugrel (0.1-1 mg/kg/day, p.o.) significantly prolongs the time to arterial occlusion and increases the duration of arterial patency in a rat model of electrically-induced arterial thrombosis. [3] |