| Description |
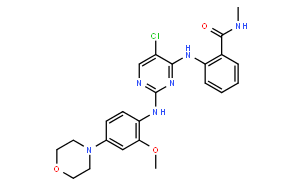
TAE226 (NVP-TAE226) is a potent FAK inhibitor with IC50 of 5.5 nM and modestly potent to Pyk2, ~10- to 100-fold less potent against InsR, IGF-1R, ALK, and c-Met. |
|
Targets
|
|
| In vitro |
NVP-TAE226 (< 1 μM) inhibits extracellular matrix-induced autophosphorylation of FAK (Tyr397) in serum-starved U87 cells. NVP-TAE226 (< 1 μM) also inhibits IGF-I-induced phosphorylation of IGF-1R and activity of its downstream target genes such as MAPK and Akt in both U87 and U251 cells. NVP-TAE226 (<10 μM) retards tumor cell growth and attenuats G(2)-M cell cycle progression associated with a decrease in cyclin B1 and phosphorylated cdc2 (Tyr15) protein expression in both U87 and U251 cells. NVP-TAE226 (1 μM) inhibits tumor cell invasion by at least 50% compared with the control in an in vitro Matrigel invasion assay in glioma cell lines. NVP-TAE226 (1 μM) treatment of glioma cell lines containing wild-type p53 mainly exhibits G(2)-M arrest, whereas glioma cell lines bearing mutant p53 undergoes apoptosis, as evidence by detection of caspase-3/7 activation and poly(ADP-ribose) polymerase cleavage and by an Annexin V apoptosis assay. [1] NVP-TAE226 (5 μM) inhibits phosphorylation of FAK in the human neuroblastoma cell line SK-N-AS. NVP-TAE226 (<10 μM) treatment of the human neuroblastoma cell line SK-N-AS leads to decrease in cellular viability, cell cycle arrest, and an increase in apoptosis. [2] NVP-TAE226 (0.1 μM-10 μM) inhibits tube formation of HMEC1 cells. [3]
|
| In vivo |
NVP-TAE226 (75 mg/kg) significantly increases the survival rate of mice bearing intracranial glioma xenografts. [1] NVP-TAE226 (100 mg/kg, oral) exerts significant decrease in microvessel density in a human colon cancer model in SCID mice. [3] NVP-TAE226 (100 mg/kg, oral) efficiently inhibits MIA PaCa-2 human pancreatic tumor growth without body weight loss in vivo model. [4] NVP-TAE226 inhibits 4T1 murine breast tumor growth and metastasis to the lung in a dose-dependent manner in vivo model, associated with inhibition of FAK autophosphorylation at Y397 and Akt phosphorylation at Serine473. [5]
|
 COA
COA MSDS
MSDS HPLC
HPLC NMR
NMR